Part a 4 the Ph Reading Is Taken Before the Ph Meter Stabilizes
The Hydronium Ion
- Page ID
- 1290
Owing to the overwhelming excess of \(H_2O\) molecules in aqueous solutions, a bare hydrogen ion has no take a chance of surviving in water.
Free Hydrogen Ions do not Exist in Water
The hydrogen ion in aqueous solution is no more than a proton, a blank nucleus. Although information technology carries only a single unit of measurement of positive charge, this charge is concentrated into a book of space that is merely nigh a hundred-millionth as large as the volume occupied by the smallest atom. (Call up of a pebble sitting in the middle of a sports stadium!) The resulting extraordinarily high charge density of the proton strongly attracts information technology to any function of a nearby atom or molecule in which there is an excess of negative accuse. In the example of water, this volition exist the lone pair (unshared) electrons of the oxygen cantlet; the tiny proton will exist buried inside the lone pair and will class a shared-electron (coordinate) bond with it, creating a hydronium ion, \(H_3O^+\). In a sense, \(H_2O\) is acting as a base here, and the production \(H_3O^+\) is the conjugate acid of water:
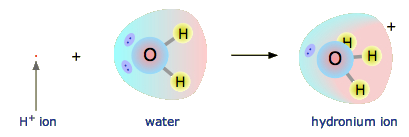
Although other kinds of dissolved ions have water molecules spring to them more than or less tightly, the interaction between H+ and \(H_2O\) is so potent that writing "H+ (aq) " hardly does it justice, although information technology is formally right. The formula \(H_3O^+\) more adequately conveys the sense that information technology is both a molecule in its own right, and is also the cohabit acid of water.
The equation "HA → H+ + A–" is so much easier to write that chemists still use it to represent acrid-base reactions in contexts in which the proton donor-acceptor mechanism does not need to be emphasized. Thus, it is permissible to talk about "hydrogen ions" and use the formula H+ in writing chemical equations as long as you think that they are not to be taken literally in the context of aqueous solutions.
Interestingly, experiments bespeak that the proton does not stick to a single \(H_2O\) molecule, but changes partners many times per second. This molecular promiscuity, a consequence of the uniquely small size and mass the proton, allows information technology to motion through the solution by rapidly hopping from 1 \(H_2O\) molecule to the next, creating a new \(H_3O^+\) ion as it goes. The overall upshot is the same equally if the \(H_3O^+\) ion itself were moving. Similarly, a hydroxide ion, which tin be considered to be a "proton pigsty" in the h2o, serves every bit a landing bespeak for a proton from another \(H_2O\) molecule, so that the OH– ion hops about in the same way.
Considering hydronium and hydroxide ions tin "movement without actually moving" and thus without having to plow their style through the solution by shoving aside water molecules, as do other ions, solutions which are acidic or alkaline have extraordinarily high electrical conductivities .
Reaction
The hydronium ion is an of import factor when dealing with chemical reactions that occur in aqueous solutions. Its concentration relative to hydroxide is a direct measure of the pH of a solution. It can exist formed when an acid is present in water or simply in pure water. It'due south chemical formula is \(H_3O^+\). Information technology tin can also be formed by the combination of a H+ ion with an \(H_2O\) molecule. The hydronium ion has a trigonal pyramidal geometry and is composed of three hydrogen atoms and one oxygen atom. There is a lone pair of electrons on the oxygen giving information technology this shape. The bond bending betwixt the atoms is 113 degrees.
\[H_2O_{(fifty)} \rightleftharpoons OH^-_{(aq)} + H^+_{(aq)}\]
As H+ ions are formed, they bond with \(H_2O\) molecules in the solution to form \(H_3O^+\) (the hydronium ion). This is considering hydrogen ions exercise not exist in aqueous solutions, merely take the form of the hydronium ion, \(H_3O^+\). A reversible reaction is one in which the reaction goes both ways. In other words, the water molecules dissociate while the OH- ions combine with the H+ ions to class h2o. Water has the ability to attract H+ ions because it is a polar molecule. This ways that it has a partial accuse, in this case the charge is negative. The partial charge is caused by the fact that oxygen is more electronegative than hydrogen. This means that in the bail betwixt hydrogen and oxygen, oxygen "pulls" harder on the shared electrons thus causing a partial negative charge on the molecule and causing it to be attracted to the positive accuse of H+ to form hydronium. Another way to describe why the water molecule is considered polar is through the concept of dipole moment. The electron geometry of h2o is tetrahedral and the molecular geometry is bent. This bent geometry is asymmetrical, which causes the molecule to be polar and have a dipole moment, resulting in a partial accuse.
An overall reaction for the dissociation of water to grade hydronium can be seen here:
\[ii H_2O_{(l)} \rightleftharpoons OH^-_{(aq)} + H_3O^+_{(aq)}\]
With Acids
Hydronium non but forms as a result of the dissociation of water, but also forms when h2o is in the presence of an acid. Equally the acid dissociates, the H+ ions bond with water molecules to form hydronium, every bit seen here when muriatic acid is in the presence of water:
\[HCl (aq) + H_2O \rightarrow H_3O^+ (aq) + Cl^-(aq)\]
pH
The pH of a solution depends on its hydronium concentration. In a sample of pure h2o, the hydronium concentration is \(one \times 10^{-7}\) moles per liter (0.0000001 M) at room temperature. The equation to discover the pH of a solution using its hydronium concentration is:
\[pH = -\log {(H_3O^+)}\]
Using this equation, we find the pH of pure water to be seven. This is considered to be neutral on the pH scale. The pH can either go upward or downward depending on the change in hydronium concentration. If the hydronium concentration increases, the pH decreases, causing the solution to become more than acidic. This happens when an acrid is introduced. Equally H+ ions dissociate from the acrid and bond with water, they form hydronium ions, thus increasing the hydronium concentration of the solution. If the hydronium concentration decreases, the pH increases, resulting in a solution that is less acidic and more basic. This is acquired by the \(OH^-\) ions that dissociate from bases. These ions bail with H+ ions from the dissociation of water to class \(H_2O\) rather than hydronium ions.
A variation of the equation tin be used to summate the hydronium concentration when a pH is given to us:
\[H_3O^+ = 10^{-pH} \]
When the pH of vii is plugged into this equation, we get a concentration of 0.0000001 M every bit we should.
Learning to use mathematical formulas to summate the acerbity and basicity of solutions can be difficult. Here is a video tutorial on the bailiwick of calculating hydronium ion concentrations:
Configuration of Hydronium in Water
It is believed that, on average, every hydronium ion is attracted to 6 water molecules that are non attracted to any other hydronium ions. This topic is withal currently nether fence and no existent reply has been found.
Problems
- Determine the pH of a solution that has a hydronium concentration of two.6x10-4M.
- Determine the hydronium concentration of a solution that has a pH of 1.7.
- If a solution has a hydronium concentration of 3.6x10-eight1000 would this solution exist basic or acidic?
- What is the pH of a solution that has 12.two grams of muriatic acid in 500 ml of water?
- Why do acids crusade burns?
Answers
ane. Remembering the equation: pH = -log[H3O]
Plug in what is given: \(pH = -\log[two.six \times 10^{-4}\;M]\)
When entered into a computer: pH = 3.6
2. Remembering the equation: [HiiiO] = 10-pH
Plug in what is given: [H3O] = ten-1.seven
When entered into a reckoner: 1.995x10-2Chiliad
3. Determine pH the aforementioned style nosotros did in question i: pH = -log[3.6x10-8]
pH = 7.4
Because this pH is higher up seven it is considered to be basic.
4. Beginning write out the counterbalanced equation of the reaction: \[HCl_{(aq)} + H_2O_{(l)} \rightleftharpoons H_3O^+_{(aq)} + Cl^-_{(aq)}\]
Detect that the amount of HCl is equal to the amount of \(H_3O^+\) produced due to the fact that all of the stoichiometric coefficents are 1.
So if we can figure out concentration of HCl we can effigy out concentration of hydronium.
Notice that the amount of HCl given to us is provided in grams. This needs to be changed to moles in order to notice concentration:
\[12.ii\;g\; HCl \times \dfrac{1 \;mol\; HCl}{36.457\; m} = 0.335\; mol\; HCl\]
Concentration is defined as moles per liter so we convert the 500mL of water to liters and get .5 liters.
\[\dfrac{0.335\; mol\; HCl}{0.5\; L} = 0.67\;M\]
Using this concentration nosotros tin obtain pH: pH = -log[.67M]
\[pH = 0.17\]
5. Acids crusade burns because they dehydrate the cells they are exposed to. This is caused by the dissociation that occurs in acids where H+ ions are formed. These H+ ions bond with water in the prison cell and thus dehydrate them to cause jail cell harm and burns.
References
- Petrucci, Harwood, Herring, Madura. Full general Chemical science Principles & Modern Applications. Prentice Hall. New Jersey, 2007.
- Marx, Tuckerman, Hutter, J. & Parrinello, Grand. (1999) The nature of the hydrated excess proton in h2o
- Petrucci, Ralph H. Full general Chemistry: Principles and Modern Applications. Upper Saddle River, NJ: Pearson/Prentice Hall, 2007. Print.
- www.youtube.com/watch?v=Y9E_ZlOqk4o
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acids_and_Bases_in_Aqueous_Solutions/The_Hydronium_Ion
0 Response to "Part a 4 the Ph Reading Is Taken Before the Ph Meter Stabilizes"
Post a Comment